India has one of the world’s worst records on tuberculosis. This column presents recommendations for how to fight it more cost effectively.
The global burden of tuberculosis (TB) owes much of its weight to India. Over 3 million, or a quarter of the world’s TB patients in 2011 were from India, with an estimated annual 300,000 deaths from TB (WHO 2012). The Revised National Tuberculosis Control Programme (RNTCP) is estimated to have saved 1.3 million lives over 1997-20061. However, the programme has been severely underfunded – for the 2012-2013 period, for instance, RNTCP was allocated Rs. 710.15 crores ($142 million approx.), which is only 2% of the total health sector budget2.
Under RNTCP, 662 district TB centres and almost 2,700 sub-district TB units work together to diagnose patients and treat them with short-course chemotherapy under a DOTS strategy34. The Indian government claims to have achieved a complete coverage of the DOTS-based TB policy since 2006, with a globally accepted target detection rate of 70% of new TB cases and treatment success rate of 85% since 2007 (GoI 2012). However, there is widespread geographical disparity in RNTCP’s success, with detection rates of less than 50% in many districts (Satyanarayana et al. 2011, GoI 2012).
Economic benefits of TB treatment
Like in other low- and middle-income countries, out-of-pocket medical expenditures in India are often large (van Doorslaer et al. 2007). A study from 2005 estimates that 40% of Indian patients seeking hospitalised care have to sell assets or borrow money to finance their medical expenditure (Sengupta and Nundy 2005). Meanwhile, an estimated 32.5-39.5 million Indians are annually pushed into poverty as a result of out-of-pocket medical expenditure5, with some suggesting the number is over 60 million6. Treatment of TB may involve a particularly large financial burden as most patients choose a private healthcare provider for their first visit (Uplekar et al. 2001).
Considering the significant economic impact of health shocks, it is important that policymakers consider the financial risk protection benefits of health interventions, such as universal provision of TB treatment. Most developing countries allocate healthcare resources based on the cost-effectiveness of an intervention, which only focuses on the health benefits such as lives saved and DALYs7 gained (World Bank 1993, Jamison et al. 2006). However, cost-benefit analysis is more comprehensive but less popular due to computational complexities involved.
A recent study by Verguet et al. (2012) attempts to strike a balance between these two methodologies. The authors develop a new technique for measuring the health and economic benefits of an intervention from a health systems accounting perspective. They estimate that a policy of universal public finance of TB DOTS treatment in India with a 100% coverage will annually save another 140 lives, avert $120,000 out-of-pocket private medical expenditure, and provide $17,000 worth of insurance against financial risk for every 1 million Indians. If capital markets (health loans) are included in the analysis, the total out-of-pocket expenditure averted and value of insurance provided can be as high as $235,000 and $300,000, respectively.
In reality, these estimated benefits present a lower bound because they leave out the externality benefits of secondary cases averted due to prompt treatment. Goodchild et al. (2011) estimate the economic value of well-being gained from RNTCP in India at $88.1 billion during 1997-2006. They find the programme very cost effective at $26 per DALY gained, with an economic return of $115 per dollar spent. Globally, Laxminarayan et al. (2009) find that the economic benefits of sustained DOTS is $129 billion in sub-Saharan Africa and $1.6 trillion in 22 high TB-burden countries worldwide, with a benefit-cost ratio of 92.
The National Strategic Plan (2012-2017) for RNTCP: A step in the right direction
The overwhelming evidence on the benefits of TB treatment may have inspired Indian policymakers into a strong scaling up effort. The annual status report of RNTCP (2013) presents a new vision for TB control in India. By 2017, the RNTCP hopes to achieve early detection of at least 90% of all types of TB cases (including drug resistant and HIV cases), successfully treat at least 90% of new and 85% of recurring cases, achieve 100% screening rate for smear positive TB cases, and establish a comprehensive framework for referral and treatment of HIV-TB patients. In addition, the new policy also plans to finance TB treatment at private care providers.
Figure 1. Per capita government RNTCP budget (in Rupees)8
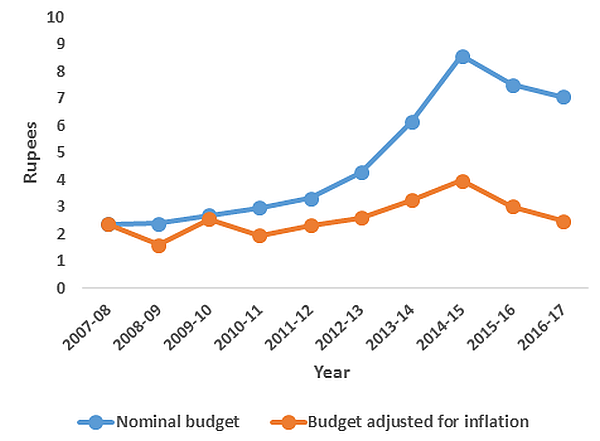 Note: Total budget spending and DOTS coverage data have been compiled from http://www.tbfacts.org and official annual status reports of RNTCP.
Note: Total budget spending and DOTS coverage data have been compiled from http://www.tbfacts.org and official annual status reports of RNTCP. The new RNTCP plan is estimated to save 750,000 lives and avert 1.7 million TB cases and 100,000 multi-drug resistant TB (MDR-TB) cases at a cost of Rs. 5825 crores ($1.17 billion) over the period 2012-2017. However, the Planning Commission of India has only approved $900 million to date. Although the total TB control budget has increased by almost threefold as compared with the 2007-2012 period (see Figure 1 for annual per capita RNTCP expenditure), it is important that this ambitious new policy is adequately and continually funded.
Need for greater focus on difficult to treat cases
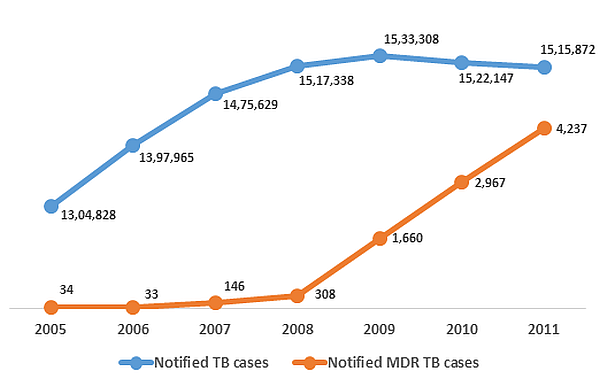
It is also time for a greater emphasis on difficult to treat TB cases. As much as 5.3% of new cases and 67% of previously-treated cases have been reported as MDR-TB in some areas of India (Mondal and Jain 2007). In addition, India recently became the third country in the world to report extensive drug resistant TB (XDR-TB) cases9. Drug resistant TB is not only difficult to treat, but also very difficult to detect, resulting in a large gap in its detection and reporting across the world (WHO 2012). In India, fewer than half of the states have reported cases of MDR-TB until now (GoI 2012). Figure 2 presents the overall notification status of TB in India.
Figure 2. Notified10 TB and MDR-TB cases in India (2005-2011)
In 2010, India chalked up a set of guidelines (called DOTS-Plus) to tackle the drug resistant TB epidemic. This new approach plans to scale up detection of MDR-TB and ensure adequate supply of drugs.
New developments
However, attention should be paid to new developments such as the Xpert MTB/RIF testing for drug resistant TB. Vadwai et al. (2011) have shown that the test can achieve a very high detection rate in India. The use of Xpert can also reduce the cost of false-positives as a percentage of total diagnostics cost from 22% to 4% (Vassall et al. 2011). Menzies et al. (2012) estimate that the Xpert test may prevent 182,000 TB deaths over a 10 year period in five southern African nations.
Although there is a large fixed cost (an Xpert machine costs approximately $17,000), donors such as UNITAID, the Gates Foundation, and USAID have recently provided monetary support to help reduce the average cost of Xpert test kits from $17 to below $10.11 With this reasonably low variable cost of running the test, India should fully embrace Xpert.
Notes:
- It has also saved 29.2 million disability-adjusted life years (DALYs); see Goodchild et al (2011).
- The total health sector budget was Rs 34,488 crores in 2012-2013. India has also received financial support for TB control from the Global Fund, DFID and USAID. The country’s total TB control budget in 2012 was $219 million (WHO 2012). We assume $1 = Rs. 50.
- DOTS stands for directly observed treatment, short-course (World Health Organization). There are five elements of the DOTS strategy – political commitment with increased and sustained financing, case detection through quality-assured bacteriology, standardised treatment with supervision and patient support, an effective drug supply and management system, monitoring and evaluation system, and impact measurement.
- Health professionals at these facilities are further aided by 13,000 microscopy centres (GoI 2012).
- See Garg and Karan (2005), van Doorslaer et al. (2006), and Bonu et al. (2009).
- See Berman et al. (2010).
- Disability Adjusted Life Years (DALYs) is a measure of overall disease burden, expressed as the number of years lost due to ill-health, disability or early death.
- For years 2012-2015 and 2015-2017, we use a total population of 1.307 billion and 1.385 billion respectively, projected by the World Bank. GDP deflator data from the World Bank has been used to estimate the real budget. For years 2012-2017, we assume that the growth rate of the deflator is the same as its average growth over 2007-2012.
- See for example an article in Nature News here.
- Reported.
- In India, the current cost of an Xpert test is $22.63 (Vassall et al. 2011).
Further reading
- Berman, P, R Ahuja, and L Bhandari (2010), ‘The impoverishing effect of healthcare payments in India: new methodology and findings’, Economic & Political Weekly, 45(16):65-71.
- Bonu, S, I Bhushan, M Rani, and I Anderson (2009), ‘Incidence and correlates of “catastrophic” maternal health care expenditure in India’, Health policy and planning, 24(6), 445-456.
- Garg, C and A Karan (2005), Equity in Asia-Pacific Health Systems, & European Commission, Health and Millennium Development Goal 1: Reducing Out-of-pocket Expenditures to Reduce Income Poverty: Evidence from India. World Health (pp. 1–25). Equity in Asia-Pacific Health Systems. Retrieved from http://www.equitap.org/publications/docs/EquitapWP15.pdf.
- GoI (2012), ‘TB India 2012: Revised National TB Control Programme Annual Status Report’, Government of India.
- GoI (2013), ‘TB India 2013: Revised National TB Control Programme Annual Status Report’, Government of India.
- Goodchild, M, S Sahu, F Wares, P Dewan, RS Shukla, LS Chauhan, and K Floyd (2011), ‘A cost-benefit analysis of scaling up tuberculosis control in India’, The International Journal of Tuberculosis and Lung Disease, 15(3), 358-362.
- Jamison, DT, JG Breman, AR Measham, G Alleyne, M Claeson, DB Evans, P Jha, A Mills, and P Musgrove (2006), Disease Control Priorities in Developing Countries, 2nd edition.
- Laxminarayan, R, EY Klein, SR Darley, and O Adeyi (2009), ‘Global investments in TB control: economic benefits’, Health affairs (Project Hope), 28(4):730-742.
- Mondal, R and A Jain (2007), ‘Extensively drug-resistant Mycobacterium tuberculosis, India’, Emerging infectious diseases, 13(9):1429-1431.
- Menzies, N, T Cohen, HH Lin, M Murray, and J Salomon (2012), ‘Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation’, PLoS medicine, 9(11), e1001347.
- Oxlade, O and M Murray (2012), ‘Tuberculosis and poverty: why are the poor at greater risk in India?’, e47533.
- Satyanarayana S, Nair SA, Chadha SS, Shivashankar R, Sharma G, et al. (2011), ‘From Where Are Tuberculosis Patients Accessing Treatment in India? Results from a Cross-Sectional Community Based Survey of 30 Districts’, PLoS ONE 6(9): e24160.
- Sengupta A and S Nundy (2005), ‘The private health sector in India’, British Medical Journal, 331:1157-1158.
- Uplekar, M, V Pathania and M Raviglione (2001), ‘Private practitioners and public health: weak links in tuberculosis control’, Lancet, 358(9285), 912-916.
- Vadwai, V, C Boehme, P Nabeta, A Shetty, D Alland, and C Rodrigues (2011), ‘Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis?’, Journal of Clinical Microbiology, 49(7):2540-2455.
- Van Doorslaer, E, O O’Donnell, RP Rannan-Eliya, A Somanathan, SR Adhikari, CC Garg, D Harbianto et al. (2007), Catastrophic payments for health care in Asia´, Health Economics, 16(11):1159-1184.
- Vassall, A, S Van Kampen, H Sohn, JS Michael, KR John,S Den Boon, JL Davis et al. (2011), ´Rapid diagnosis of tuberculosis with the Xpert MTB/RIF´ in D Wilson (ed.), High burden countries: a cost-effectiveness analysis, PLoS medicine, 8(11), e1001120.
- Verguet, S, R Laxminarayan, and DT Jamison (2012), ´Universal Public Finance of Tuberculosis Treatment in India: An Extended Cost-Effectiveness Analysis´, Disease Control Priorities in Developing Countries, 3rd Edition Working Paper No.1.
- WHO (2012), ´Global Tuberculosis Report 2012´, World Health Organization.
- World Bank (1993), ´World Development Report: Investing in Health´, Oxford University Press.




 27 May, 2013
27 May, 2013 




Comments will be held for moderation. Your contact information will not be made public.